Ammonia as a marine fuel
2023-01-20 23:51
Ammonia has attracted wide interest as a source of zero emission fuel for shipping. Almost all ammonia in use today is made from hydrocarbons, and as such confers almost no carbon abatement advantage, while simply adding costs. By contrast, green ammonia – produced by electrolysis powered by renewables or nuclear – is an excellent source of zero-emission fuel, provided that associated NOX emissions are managed appropriately. However, green ammonia is currently only produced in negligible amounts, and a massive investment programme would be required not only to produce a meaningful supply of green ammonia, but to drive down the costs of doing so, such that the fuel becomes financially viable for the shipping industry.
There is a large variety of other alternative fuels that can be used in shipping, such as Liquefied Natural Gas (LNG), methanol, dimethyl ether, ethanol, Liquefied Petroleum Gas (LPG), biodiesel (renewable diesel, FAME based biodiesel or diesel from Fischer-Tropsch-related processes), electricity, liquefied biogas (LBG), hydrogen and nuclear power. So far, LNG is the most used alternative fuel.
Ammonia has the key benefit of being easier to store than hydrogen, i.e. nearly identical to propane (LPG) at low pressure under ambient conditions. Hence, the cost of storage per energy unit is significantly cheaper than either hydrogen, electricity in batteries or LNG. Furthermore, ammonia does not contain carbon and processes for producing it from renewable energy are known, even though it typically is produced in industrial processes from natural gas. Therefore, it is possible to regard ammonia as an energy carrier that is more convenient than hydrogen, but still CO2 emission free under the right circumstances. For the purposes of this paper, CO2 emission-free ammonia from renewable electricity is labelled green ammonia, whereas ammonia from fossil sources like natural gas and coal is labelled brown ammonia. Ammonia from fossil sources with carbon capture and storage (CCS) is labelled blue ammonia.

There are, however, drawbacks with ammonia such as toxicity, limited experience as a fuel in combustion engines and low energy utilization rate that calls for a further analysis.
WHAT IS AMMONIA?
Ammonia (NH3) is a colorless gas under ambient conditions with a lower density than air. The boiling point is -33.3°C and hence by applying a moderate pressure it can be handled as a liquid at room temperature. At pressures above 8.6 bar at 20°C, ammonia is a liquid with a density of 0.61 t/m3. At the boiling point, the density is 0.68 t/m3. The heating value for ammonia on a lower heating value basis is 18.6 MJ/kg. Thus, compared to MGO the energy content is less than half on a mass basis and about 30% on a volume basis in liquid state.
When ammonia is stored under pressure in Type C tanks, the volumetric energy density can be compared to MGO, LPG and hydrogen. In addition to the density of the fuel, there will also be a penalty in volume related to the cylindrical shape of the tank and the penalty will be larger if an insulation system is required. In spite of the lower energy content for ammonia per ton compared with hydrogen, the density of the fuel results in less volume required to store the same energy for ammonia compared with hydrogen. This is especially the case for compressed hydrogen, but also for liquid hydrogen. Compared with MGO and LPG, the space requirement is significantly higher.
FACTS ABOUT AMMONIA PRODUCTION AND UTILIZATION
As mentioned above, ammonia (NH3) is known as a colorless and pungent-smelling gas at room temperature with a compound of nitrogen and hydrogen. Pure ammonia is hygroscopic and easily dissolved in water and humidity. Ammonia is, however, corrosive due to its alkaline properties. Having said that it is one of the most commonly produced industrial chemicals around the world. More than 75% of the ammonia produced is used in the agriculture sector as a fertilizer. Ammonia can also be used as a working fluid in a refrigeration cycle. The other common use of ammonia is in household cleaning solutions. Figure 1 illustrates the amount of ammonia produced around the world.
The data in Figure 1 between 1945 and 2017 (given as blue line) have been acquired. Globally, about 146 million metric tons of ammonia was produced in 2019. Only a small portion of ammonia (approximately 4%) is used in direct applications; the remaining portion is used as a chemical in industrial applications or fertilizer in agriculture. It is expected to increase the ammonia consumption with the use of ammonia in the energy sector due to environmental concerns and attempts in reducing CO2 emissions. There are many attempts all around the world in order to use ammonia as a carbon-free fuel. Many countries have announced many ammonia-related projects for power generation, off-grid application, internal combustion engines, etc. Additionally, it will be expected to increase with the increasing hydrogen-powered systems since ammonia is a good hydrogen carrier. We foresee that the production and consumption of ammonia will be increasing exponentially. Our projection is illustrated in Figure 1 with a red-dotted line. In 2050, globally about 1.2 billion metric tons of ammonia is going to be produced and increase exponentially. It is nearly 8.2 times more than the amount of ammonia produced in 2019.
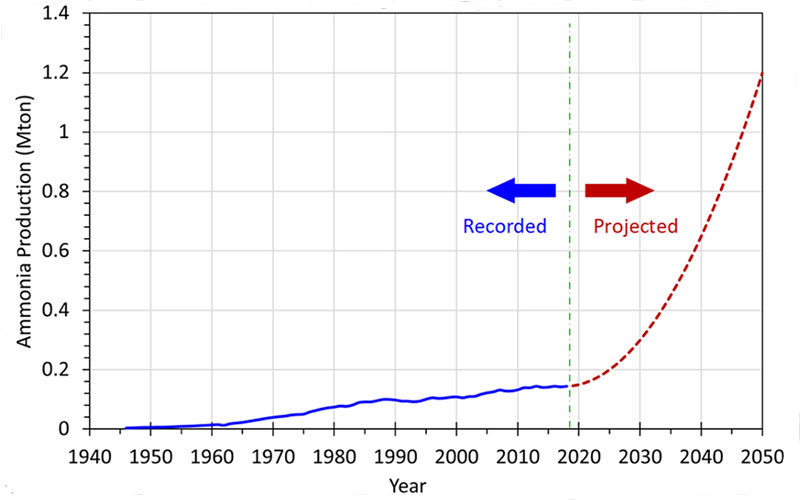 Figure 1: The amount of ammonia produced around the world
Figure 1: The amount of ammonia produced around the world
Ammonia could be the key to finalize the increasing search for an alternative fuel as it can be produced with renewables. Ammonia is a chemical that can be used in many sectors and for many different purposes. It can serve as a fuel in energy production, a key chemical in feedstock and chemicals production, the main ingredient for cleaning materials, a fuel for engines, and a refrigerant for cooling systems. Its versatile and wide range of application potential makes ammonia an important, carbon-free alternative. Some key advantages of utilizing ammonia as a clean alternative are listed as follows:
- Ammonia has three hydrogen atoms and one nitrogen atom and can flexibly be produced with conventional or renewable resources.
- It is a potential hydrogen storage and carrier.
- Transportation of ammonia is much safer compared to hydrogen.
- When liquefied, it contains approximately 48% more hydrogen by volume than hydrogen.
- No carbon dioxide emissions are emitted during its use since it is carbon free.
- It can be utilized for a wide range of applications as a fuel, working fluid, refrigerant, hydrogen carrier, fertilizer, feedstock, chemical, cleaning agent, and many more.
- It can be easily detectable when any leakage occurs because of its distinctive smell.
- A strong fuel candidate for engines, gas turbines, power generators, and burners. The modifications needed for such engines are relatively small.
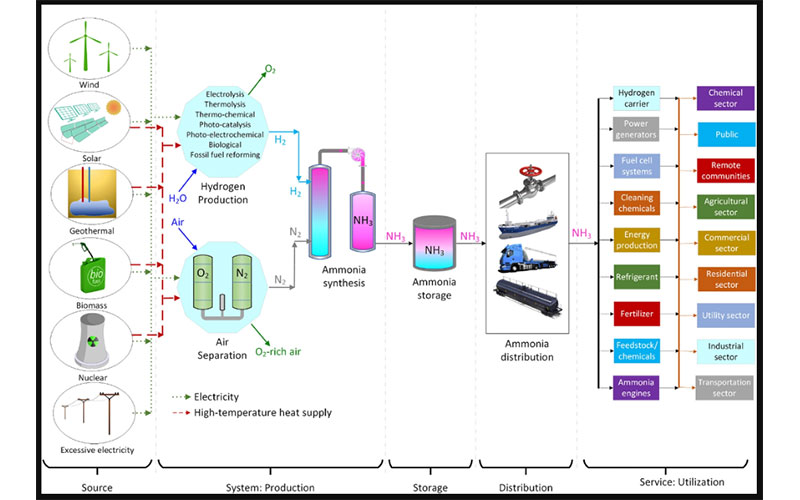 Figure 2: An economic cycle of ammonia from production to utilization
Figure 2: An economic cycle of ammonia from production to utilization
[Color figure can be viewed at wileyonlinelibrary.com]
Although ammonia has widely been used for many years in the refrigeration systems as a refrigerant and the production of fertilizers, household cleaning chemicals, and disinfectants, it has started regaining attention from researchers, scientists, engineers, and technologists due to its carbon-free nature which can be used as a potential fuel to reduce the CO2 emissions. It can play a critical role in solving various challenges related to hydrogen energy options and hydrogen economy as a unique hydrogen storage medium (with three atoms of hydrogen) and transportation and distribution, without changing the infrastructure, with the existing transportation and distribution options where industries currently deploy. In the last decade, the attempts have considerably increased to use ammonia in internal combustion engines and gas turbines.
In Table 1, the common fuels used in internal combustion engines are given with their combustion properties.
Numerous important advantages of ammonia as a potential fuel can be listed as follows:
- It is carbon free and environmentally benign.
- It has three atoms of hydrogen and may potentially be used as hydrogen carrier.
- Its production, storage, transportation, and distribution are much easier and less complicated than many other fuels.
- It is cost-effective and economically feasible for applications.
- It can be considered a potential replacement for gasoline, diesel, and kerosene.
- It can be considered for all combustion systems, ranging from engines to gas turbines.
- It can be a potential fuel solution for clean power generation in remote areas.
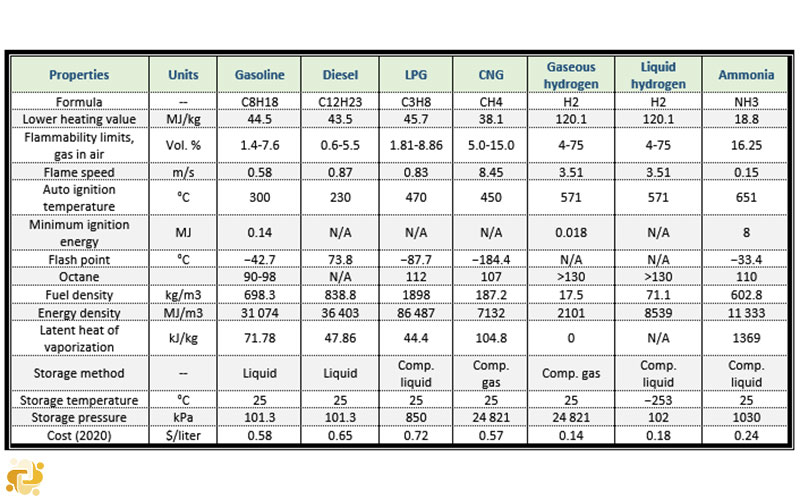 TABLE 1. Comparison of common fuels used in internal combustion engines
TABLE 1. Comparison of common fuels used in internal combustion engines
A comparison of various types of fuels with ammonia is given in Table 1. Although energy density of ammonia in pressurized tank is 2.5 times lower than gasoline, it has a big advantage according to traditional fuels in terms of the specific energetic cost. Also, when ammonia is produced with renewables, the life cycle costs of ammonia-fuel blend–driven systems can be reduced substantially.
CHALLENGES WITH AMMONIA COMBUSTION
It is important to point out that ammonia, when used for combustion, has some side effects, just like the way all drugs have some side effects. Such side effects (so-called: challenges) may be listed as follows:
- High ignition temperature
- Low flame velocity
- Slow chemical kinetics
A challenge for internal combustion engines is the possibility of unburned ammonia in the exhaust, that is toxic and highly pungent even in small amounts. If this occurs during normal running operation, it may be mitigated by an ammonia catch system, like a water curtain. However, if the level of ammonia slip is the same as or lower than the NOX emissions, this can be mitigated in an SCR. Also, it has been suggested that ammonia slip may cause reduction of NOX in the expansion stroke.
SOLUTIONS TO THE CHALLENGES
High ignition temperature:
In order to solve the difficult ignition problem of ammonia, it is a common way to mix ammonia with traditional fuels using in internal combustion engines such as gasoline, diesel, LPG, CNG, ethanol, methanol, hydrogen, etc. Figure 3 illustrates the use of ammonia-fuel blends in internal combustion engines. Ammonia can be taken into the engine either with air in its gas form from the air intake manifold or injecting to the cylinder in its liquid form separately from accompanying fuel. Since the traditional fuels will ignite at a lower temperature, it will increase the temperature of the cylinder and it helps to ignite ammonia.
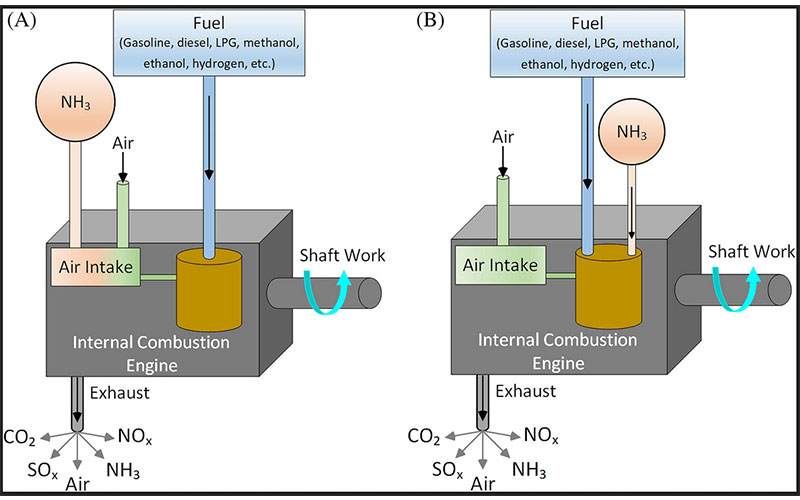 Figure 3: Illustration of ammonia-fuel blend–driven internal combustion engine: (A) ammonia intake with air in the gas form and (B) ammonia intake separately with fuel
Figure 3: Illustration of ammonia-fuel blend–driven internal combustion engine: (A) ammonia intake with air in the gas form and (B) ammonia intake separately with fuel
[Color figure can be viewed at wileyonlinelibrary.com]
Ammonia-fuel blends can reduce the need for additional devices or modifications in engines. Thus, the transition to the hydrogen economy can be achieved cost-effectively in internal combustion engines. The common way to send the ammonia into the engine is to send with air in gas form from the air intake manifold (Figure 3A). In order to achieve as possible as maximum power and as possible as the lowest emissions, the ammonia, fuel, and air mixture should adjust carefully. This requires a comprehensive blender design and automatic control system. Therefore, an optimum blender should be designed for each conventional fuel to be mixed with ammonia to adjust the optimum mixing ratio for ammonia, fuel, and air. Ammonia can also be taken in by injecting ammonia and fuel separately into the intake manifold in the liquid phase, as seen in Figure 3B. In this method, the flow rates of ammonia and fuel should be well adjusted. Also, effective ammonia injectors should be developed to prevent ammonia slip.
On the other hand, ammonia-fuel blends cause power reduction due to ammonia combustion. Yapicioglu and Dincer have performed a comprehensive study to investigate the effective ammonia-fuel blends in a power generator engine. Figure 4 demonstrates the effect of changing the ratio of ammonia fuel blends on power output. As expected, the power output from the generator has reduced with increasing the ratio of ammonia in the fuel mixture. Also, the highest power output has been obtained by ammonia-hydrogen blend. Additionally, the exhaust temperature tends to decrease with increasing the ratio of ammonia in the fuel mixture. While the ratio of ammonia in fuel blend is changing from 0.20 to 0.80, the exhaust temperature has reduced about 60°C in diesel, 150°C in hydrogen, and 100°C in propane and natural gas blends.
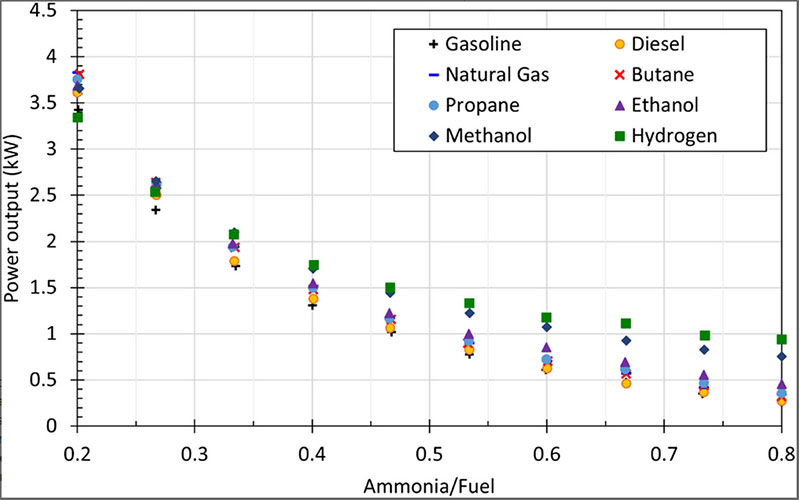 Figure 4: The effect of varying the ratio of ammonia fuel blends on the power output of power generator
Figure 4: The effect of varying the ratio of ammonia fuel blends on the power output of power generator
[Color figure can be viewed at wileyonlinelibrary.com]
In order to enhance the combustion processes and power output, a supercharger can be integrated into the engine. The engine can be more excessively supercharged than traditional engines due to high octane rating of ammonia. Higher compression ratios can also help to solve difficult ignition problem. Increasing pressure will increase the temperature of the fuel mixture in the cylinder. Thus, the combustion can occur more easily. Preheating for ammonia can also help the auto ignition. Higher temperature ammonia intake can help enhance the initiation and progression of combustion.
On the other hand, the accompanying fuel causes CO2 and NOx emissions. When emissions for the engines fueled by traditional fuels and ammonia-fuel blends are compared, ammonia provides a significant advance to reduce emissions. Here, the use of hydrogen in the ammonia-fuel blend suppresses the carbon emissions since the fuel mixture does not contain carbon compounds. Ammonia-hydrogen blend is also significant for reducing carbon emissions as there is no carbon in the mixture. The effect of ammonia-fuel blends on CO2 and NOx emissions is shown in Figure 5, as expressed in the next subsection.
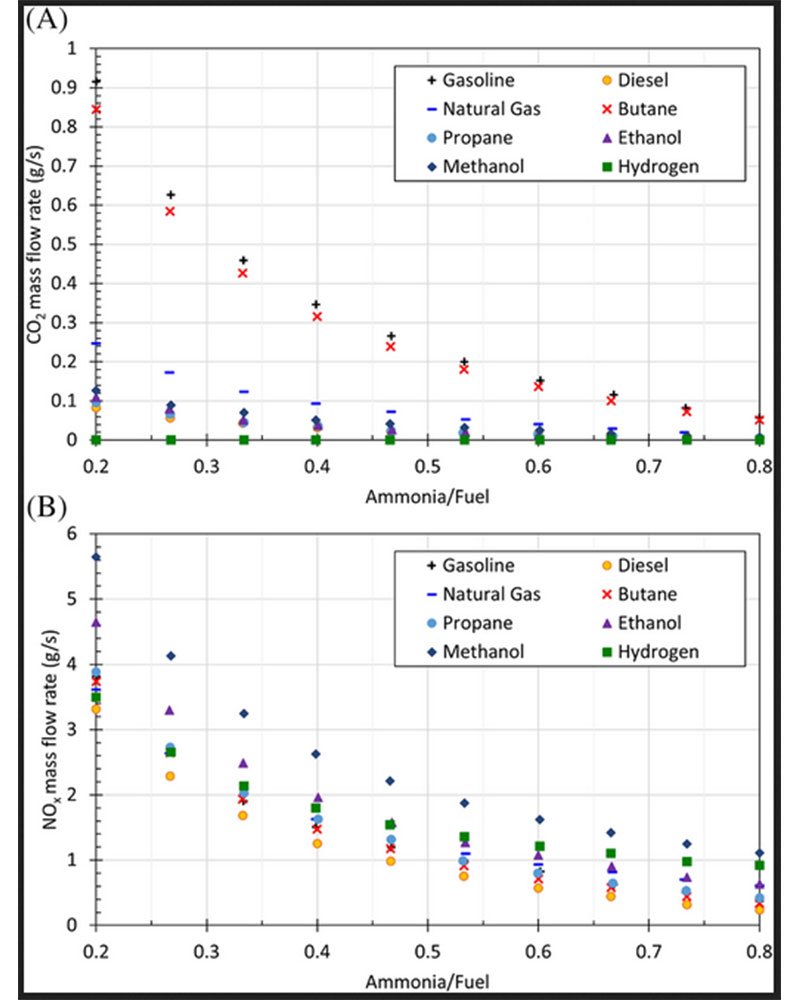 Figure 5: (A) CO2 and (B) NOx emissions for different ammonia fuel blends
Figure 5: (A) CO2 and (B) NOx emissions for different ammonia fuel blends
[Color figure can be viewed at wileyonlinelibrary.com]
Low flame velocity
The second challenge for the use of ammonia in an internal combustion engine is the lower flame speed of ammonia according to other traditional fuels. The lower flame speed restrains temperature diffusion in the cylinder during the combustion stroke and causes the power reduction. This problem is seen in both spark ignition and compression engines. Consequently, the use of ammonia in a conventional engine is not possible to use without power losses. However, when it is taken into consideration that being carbon-free fuel and its potential to reduce carbon emissions, ammonia is still a worthwhile and significant alternative fuel for internal combustion engines. All the solutions described above can also help in resolving low flame velocity. Namely, ammonia-fuel blend, preheated ammonia, and higher compression ratios can improve flame velocity.
Ammonia can be used in spark ignition engines by injecting ammonia and gasoline separately into the intake manifold in liquid phase. As ammonia combusts one-fifth time slower than gasoline, the spark timing requires special arrangement of the crank angle/piston position. When 70% of gasoline is substituted into ammonia, same amount of carbon dioxide is reduced. Ammonia should not be injected into the cylinder unless pressure is higher that cylinder compression pressure.
In the compression ignition engines, ammonia is premixed with the air and introduced through the intake manifold and small quantity of diesel fuel or another promoter is injected inside the cylinder to have the ammonia-air mixture ignited. As mention before, here, it is important to adjust and auto control mixture ratios of ammonia, fuel, and air is a significant issue.
Slow chemical kinetics
The chemical reaction rate is the speed at which a chemical reaction occurs. It is also defined as the speed at which the reactant converted into the products. When ammonia is used in internal combustion engines as a fuel, the chemical reaction rate is slow than traditional fuels due to its high ignition temperature and low flame velocity. This slow chemical reaction rate causes ammonia to be discharged from the exhaust without burning. The common way to enhance the chemical reaction rate of ammonia combustion is to use the promoter in ammonia-air mixture. Traditional fuels and hydrogen are commonly used as a promoter in ammonia-fueled engines, as expressed in the previous section. Ammonia-fuel blends can increase the chemical reaction rate of ammonia combustion. Also, NaCl, BaCL2, and NaF are used for a catalyst to enhance chemical kinetics of ammonia combustion.
CO2 AND NOx EMISSIONS
Figure 5 shows the CO2 and NOx emissions for different ammonia-fuel mixtures with respect to the mixture ratio. It is clear that increasing the amount of ammonia in the mixture substantially reduces CO2 and NOx emissions. In pure ammonia and ammonia-fuel blends, especially ammonia-hydrogen mixture–driven combustion engines, NOx emissions are a critical problem. In order to prevent this problem, ammonia oxidation catalyst and catalytic reduction can be used. Thus, both NOx and NH3 can be reduced. The use of ammonia-hydrogen blend can eliminate carbon emissions. Also, enhancement of ammonia combustion can reduce NOx emissions.
CURRENTLY AVAILABLE TECHNOLOGIES
There are a number of conversion processes for ammonia as a fuel. Ammonia has been considered as a fuel in fuel cells, primarily solid oxide fuel cells (SOFC) since a PEM fuel cell would require cracking of ammonia and is very sensitive to ammonia impurities. Research on SOFC has been carried out, but there are no such commercially available fuel cells.
Internal combustion engines are considered a better option than fuel cells in the near future because of cost, power density, load response and robustness.
For two-stroke engines, MAN ES has developed the dual fuel engines series ME-GI and ME-LGI that have been developed to address a number of new fuels including LNG, methanol, LPG and ethane. Ammonia is considered to be a more challenging fuel, and it is expected that pilot fuel like diesel would be required and possibly a larger amount than for LPG. MAN ES has started to develop two-stroke engines for ammonia, which likely will be based on the ME-LGI engine used for LPG, and are expected to be able to be delivered up to 2024.
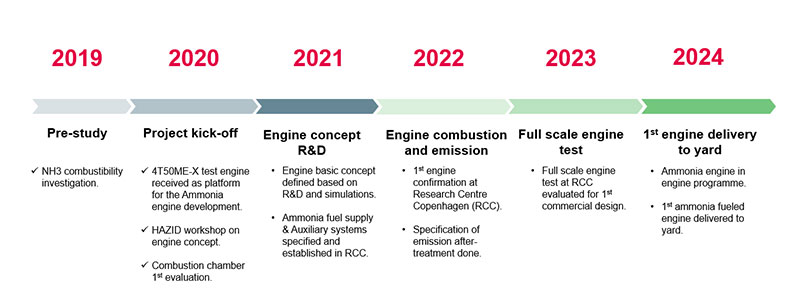 Figure 6: Two-stroke Ammonia engine development schedule by MAN energy Solutions
Figure 6: Two-stroke Ammonia engine development schedule by MAN energy Solutions


